Various parameters are evaluated for the validation of HVAC system or cleanroom. Here we list the top criteria to select a service provider for HVAC or cleanroom validation.
Cleanroom validation or HVAC validation is an essential part in pharmaceutical, biotechnology, hospitals, aerospace, automotive and semiconductor office usefulness.
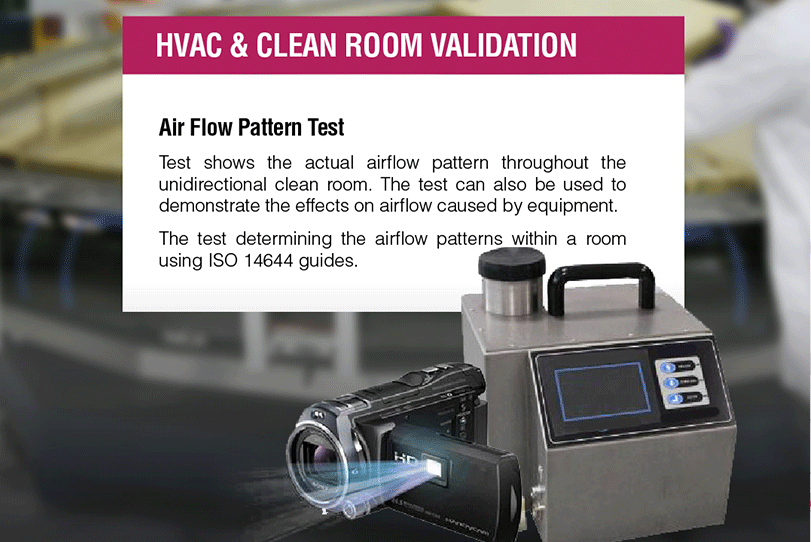
Approval of HVAC or cleanroom is a fundamental liable to give recorded proof about the exactness of results created by it. The different boundary to be assessed for HVAC approvals are light Intensity test, air flow pattern test, sound level measurement, particle count test, recovery test, HEPA filter integrity test, and air velocity test.
Validation is a significant cycle for any cleanroom and HVAC systems, it serves to guarantee that the cleanroom is appropriately introduced and intended for its proposed ISO arrangement and that the entirety of the parts (office, climate, hardware) meet administrative necessities and other characterized guidelines, especially during COVID-19 scenario approval is prime significance.
Conducting a series of tests to qualify if a controlled environment is performing in accordance with the process requirements and the applicable regulatory guidelines.
Standards/Guidelines
• ISO-14644-1, 2, 3
• EU-GMP Annex-1
• USFDA
• Schedule M (India).
HVAC system validation Tests:
• Airflow velocity and Air changes per hour
• Filter leak test/ Filter integrity test (PAO Test)
• Non-Viable Particle count / Particles per cu.mt volume
• Room air change per hour (ACH) / Fresh air determination
• Room pressurization test
• Recovery
• Airflow pattern
• Temperature and humidity uniformity test.
Validation of Lab Instruments
• Bio-safety Cabinet
• Laminar Airflow unit
• Reverse Laminar Airflow unit
• Dynamic Pass box.
Bio-safety Cabinet Validation Tests
• Downflow Velocity Test.
• Inflow Velocity Test.
• Filter leak test/ Filter integrity test (PAO Test)
• Non-Viable Particle count
• Airflow pattern
• Light/Lux Test.
• Noise Level Test.
With the growth of end-user industries, manufacturers need the expert help of labs offering calibration services which can analyse the equipment in detail and certify them. But how can one be sure of the selection criteria of the labs themselves?
Top 7 criteria to select your HVAC or /cleanroom validation service provider in India
1. ISO17025 licensed labs and these accreditations are a characteristic of value and standard for alignment labs.
2. Customised benefits according to client’s prerequisite besides performing alignment according to the necessary norms.
3. HVAC validation and calibration exactness the primary objective for any specialist co-op is to limit any estimation vulnerability by guaranteeing the precision of test gear and offer elite estimation in adjustment.
4. Best-in-class advanced facilities. Are service supplier’s labs are outfitted with cutting-edge testing gear, programming and cutting-edge foundation and with an ever-increasing number of mechanical headways happening occasionall?.
5. Experienced and trained technicians because there is no supplanting for prepared workers with state-of-the-art information on the adjustment cycles and strategies.
6. Trust and brand name.
7. Customer-centric approach. One of the major challenges that service providers currently face is to become more customer-centric. Godrej Calibration services are happy and feel proud to mention that over 85% of our business comes from repeat customers which reflects the high-quality services and customer satisfaction level year on year.
Godrej Calibration Services is constantly refreshing and extending its HVAC or cleanroom validation and calibration abilities to meet its clients’ necessities. Their labs have presence in all major cities in India including Mumbai, Chennai, Vadodara, Pune, Gurgaon, Hyderabad, Bangalore, Vizag, and Bhopal.
Authored by:
Ms. Parameswari Kennedy, Head, Godrej Calibration Services
Cookie Consent
We use cookies to personalize your experience. By continuing to visit this website you agree to our Terms & Conditions, Privacy Policy and Cookie Policy.